Proceedings of The World Avocado Congress III, 1995 7-10
PH REGULATION OF PHOTOSYNTHETIC PEPC FROM AVOCADO FRUIT
World
Avocado Congress III, October 22-27, 1998:7-10.
Michael
M. Blanke
Institut
für Obstbau und Gemüsebau der Universität Bonn
Auf
dem Hügel 6
D-53121 Bonn
Germany
Brian
A. Notton
Institute
of Arable Crops Research
Long
Ashton Research Station
Long
Ashton
Bristol BS18 9AF
England
Additional
key words: C02-assimilation, C02-refixation, respiration.
Abstract
Phosphoenolpyruvate
carboxylase, PEPC, assimilates or recycles C02 within the fruit and
appears to be a key enzyme in avocado fruit photosynthesis. PEPC was extracted from
cv. Fuerte avocado fruit and was purified by ammonium sulfate precipitation,
gel filtration and hydroxylapatite chromatography and found to consist of two
isoenzymes. Regulation of enzyme activity in the avocado fruit was primarily by
activation of the glyolytic intermediate glucose-6-phosphate and end-product
inhibition by L-malate in a strongly pH-dependent manner.
1.
Introduction
The large energy requirement of the avocado fruit,
mostly for its oil synthesis, is partially accounted for by a high respiration
rate relative to other fruits (Blanke1991).
Phosphoenolpyruvate
carboxylase [PEPC, EC 4.1.1.31] catalyses the carboxylation of
phosphoenolpyruvate (PEP) with bicarbonate (HCO3) (Notton and Blanke,
1992 and 1993). PEPC is a key enzyme in fruit photosynthesis (Blanke
and Lenz, 1989) where it (re-) assimilates C02/HCO3
within the fruit and has not yet been purified from any fruit. Regulation of
PEPC activity in avocado fruit is by two effectors, inhibition by L-malate and
stimulation by glucose-6-phosphate (Blanke and Notton, 1991; Notton and Blanke,
1993).
The objective of the present work is to investigate the pH-dependence of this regulation and purify the enzyme from avocado fruit.
2.
Materials and methods
2.1 - Enzyme extraction
Pericarp of ripe avocado cv.
Fuerte was diced and extracted by grinding in a pre-cooled pestle and mortar in
sand using 50 mM Tricine-NaOH at pH 7.8, containing 5 mM MgSO4, 5 mM
NaHCO3, 15 mM DTT, 20 mM ascorbate and 5 % (w/w) PVP. The homogenate
was filtered through muslin, centrifuged at 20,000 g for 30 mints and floating
oils removed.
2.2 -_Purification of PEPC
PEPC was precipitated between 30% and 50% saturated ammonium-sulfate. The resultant pellet was dissolved in 50 mM MOPS buffer pH 7.0, containing 5mM DTT, and the solution desalted on a Sephadex G-25 column and applied to spheroidal hydroxylapatite. Unbound protein was removed by washing the column with the MOPS buffer and PEPC eluted by a stepwise inorganic phosphate gradient from 0 to 150 mM Pi. The 50mM Pi in MOPS fraction contained 70-80% of the PEPC and was made 50% saturated with ammonium sulfate to precipitate the PEPC, the pellet redissolved in MOPS buffer and the solution stored at -20ºC. This procedure resulted in a 25-fold enrichment of the PEPC with a recovery of 50% of the initial activity.
2.3 - PEPC assay
The initial rate
of PEPC was measured as described by Notton and Blanke (1993) by coupling the
reaction to the oxidation of NADH in the presence of MDH, and adding malate,
and glucose-6-phosphate as required for the particular experiment.
3.
Results and Discussion
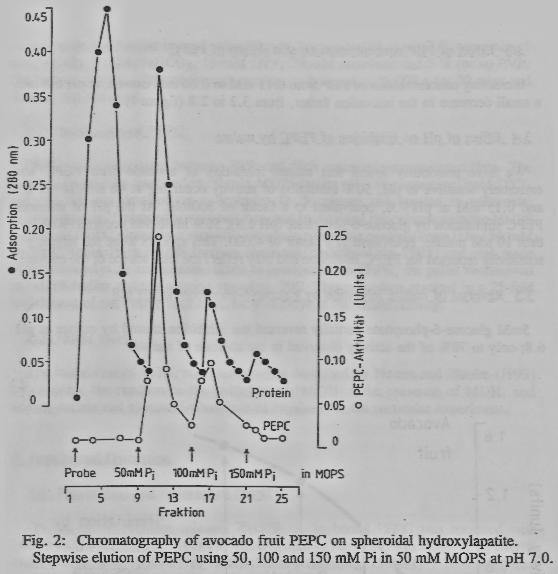
3.1 - Purification and isoforms-of PEPC
On the hydroxylapatite
column, 70-80% of the bound PEPC was removed with MOPS buffer containing 5OmM
Pi, the remainder of the PEPC was removed by MOPS buffer containing 100mM Pi.
MOPS buffer containing 150mM Pi removed more protein but no PEPC (figure2).
This suggests the presence of two isoforms of PEPC in avocado fruit which
confirms reports of PEPCs from leaves (Notton and Blanke, 1993).
The 5OmM Pi in
MOPS fraction was made 50% saturated with ammonium sulfate to precipitate the
PEPC, the pellet redissolved in MOPS buffer and the solution stored at -20C.
This procedure resulted in a 25-fold enrichment of the PEPC with a recovery of
50% of the initial activity. To our knowledge, this is the first purification
of PEPC from a fruit.
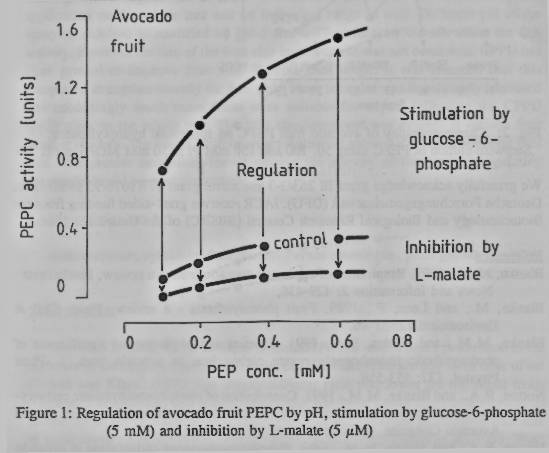
3.2 Effect of pH on stimulation of PEPC
by glucose-6-phosphate
The response of
avocado PEPC to 5mM glucose-6-phosphate, over the pH range 6-9 showed that
stimulation of activity occurred only in the region of pH 7. An examination of
this effect over the narrow range of pH 6.3-7.5 showed that stimulation of PEPC
occurred only between pH 6.5 and 7.0 with a maximum at pH 6.8 and no
glucose-6-phosphate stimulation at pH 7.8.
3.3 Effect of PEP concentration on
stimulation of PEPC
Increasing concentrations of PEP from 0. 11 mM to 0.66
mM caused, at pH 6.8 only a small decrease in the activation factor, from 3.2
to 2.8 (figure 1).
3.4 -Effect of pH on inhibition of PEPC
by malate
We have
previously found that malate inhibition of avocado fruit PEPC was extremely
sensitive to pH, 50% inhibition of activity occurring at 46 MM at pH 8.0 and
0.15 mM at pH 7.0, equivalent to a factor of 300fold. At the pH of maximal PEPC
stimulation by glucose-6-phosphate (pH 6.8), 50% inhibition occurred with less
than 10 /μM malate, equivalent to a factor of 4,600. This appears to be
the largest pH sensitivity reported for PEPC inhibition and may reflect the
fruit source of the enzyme.
3-5 -Reversal of malate inhibition by glucose-6-phosphate at pH 6.8
5mM
glucose-6-phosphate partially reversed the inhibition caused by malate at pH
6.8; only to 70% of the activity observed in the absence of malate.
We gratefully acknowledge
grant B1 263/3-3 and travel grant 477/1076/95 to MMB of Deutsche Forschungsgemeinschaft
(DFG). IACR receives grant-aided funding from the Biotechnology and Biological
Research Council (BBSRC) of the United Kingdom.
References
Blanke, M. M., 199 1.
Respiration of apple and avocado fruits - a review. Postharvest - News and
Information 2: 429-436.
Blanke, M., and Lenz, F.,
1989. Fruit photosynthesis - a review. Plant, Cell & Environment 12: 31-46.
Blanke, M.M., and Notton,
B.A., 1991. Kinetics and physiological significance of photosynthetic
phosphoenolpyruvate carboxylase in avocado fruit. J. Plant Physiol. 137:
553-558.
Notton, B.A., and Blanke,
M.M., 1993. Phosphoenolpyruvate carboxylase in avocado fruit: Purification and
properties. Phytochemistry 33: 1333-1337.