Proceedings
of The World Avocado Congress III, 1995 185 - 188
BORON UPTAKE BY CONTAINER-GROWN, ROOTSTOCK AVOCADO
PLANTS FROM DIFFERENT
BORON-CONTAINING MEDIA
P.J. Robbertse E. Tomer
Department of Plant Production Volcani
Institute
and Soil Science Bet-Dagan
University of Pretoria Israel
Pretoria, 0002
South Africa
L.A. Coetzer
R.O. Barnard
Department of Botany Department
of Plant Production
University
of Pretoria
and Soil Sciences
Pretoria 0002 Pretoria
0002
South Africa South Africa
Abstract
Working with the influence of
boron on fruit set in avocado, we were interested in boron uptake and the localisation
of boron in different plant organs. 'Hass' avocado plants on clonal 'Duke 7'
rootstocks were grown in containers filled either with washed sand or soil (+
or - humic acid), in a temperature controlled glasshouse. A 50% Hoagland's
solution with varying boron levels was applied and leaves, roots and stems were
analyzed at regular intervals for total dry mass and boron concentrations.
On average there was a linear
increase in the boron content of all three organs (roots, stems and leaves),
with increasing boron levels of the medium, reaching higher levels in the
plants growing in the soil + humic acid, and lowest levels in sand. The dry
mass of the plants growing in the sand was much higher than of those in the
soil and on average, showed a linear decrease with increments in the boron
concentration of the medium. The highest dry mass of all organs and best
general growth was obtained in the sand culture with 0.5 mg/f boron. Severe
toxicity symptoms appeared at higher boron concentrations. Boron concentrations
of leaves from plants growing in the soil + humic acid, increased to more than
900 mg/kg. It seems very difficult to control boron concentrations in avocado
leaves by applying boron through the roots.
1. Introduction
The
importance of boron as an essential micro-element and in particular for pollen
tube growth, has become a fact (Vasil 1963). For optimal pollen tube growth to
occur in avocado pistils, a boron concentration of between 50 mg/kg and 100
mg/kg in the flowers is required (Robbertse & Coetzer 1988). Using boric
acid enriched with the isotope 11B, Coetzer et al. (1993) showed that during
flowering, boron is translocated from the youngest mature avocado leaves to the
inflorescences. To obtain a concentration of 100 mg/kg in the flowers, the
boron concentration in the young, mature leaves should be above 70 mg/kg.
Although we did manage to get close to these levels by spraying Solubor on the
leaves (Robbertse & Coetzer 1990; Robbertse et al. 1992), virtually nothing
is known about boron uptake by avocado roots. This paper reports on the results
of experiments on boron uptake by avocado roots from different boron-containing
substrates and localisation in the roots, stems and leaves.
2. Materials and methods
2. 1. Sand culture
Avocado plants for this
experiment were first grown in a water medium, but for some unknown reason the
most of them died after a month (Coetzer et al. 1994) and we had to switch to
the sand culture. Sixty young 'Hass' avocado trees (on average 1.0 in high),
grafted on 'Duke 7' clonal rootstock were planted in 5 liter containers filled
with washed sand. For the first 9 weeks the plants received a daily drench with
a 50% Hoagland's solution without boron. After this period the plants were
separated into five groups of 12 plants each. The twelve control plants
continued to receive the zero boron and 50% Hoagland's, while for the other
four groups, either 0.5 mg/l boric acid (BA), 1.0 mg/l BA, 2.5 mg/l BA or 5.0
mg/I BA was added to the Hoagland's. The plants were kept on a rotating table
in a temperature controlled greenhouse (15ēC/28ēC). Four plants from each
treatment were harvested four, eight and twelve weeks after the boron
applications were started and the following parameters recorded: Plant height;
diameter of stem; number of leaves; total dry mass of roots, leaves and stem;
boron concentration of dry roots, stems and leaves.
2.2. Soil-medium
Sixty plants, as for the sand
culture, were planted in a soil mixture (pH 5.8) of coarse sand, top soil and
compost (25:50:25). All plants received a drench with distilled water and one
soak with 50% Hoagland's without boron. The plants were grouped into five times
12 plants each. The 12 "control" plants did not receive boron while,
based on previous experience, the other groups either received 0.25 g Solubor,
0.75 g Solubor, 1.50 g Solubor or 3.00 g Solubor per container. The Solubor was
dissolved in 500 nil distilled water and applied as a drench, once only, four
weeks after planting when the young trees had settled down. Six plants from
each of the five treatments, in addition, received a single treatment with
humic acid (3 g dry powder, mixed with the upper soil layer). The same
parameters were recorded as for the sand culture.
All results were
statistically analyzed using a Genstat 5 programme.
3. Results
The results are presented in
figures I to 4. Figures are adjusted means, using plant
height of the first harvest
as co-variant. Highly significant differences were found between the plant
heights of the different treatments. The two soil treatments gave very similar
results and therefore figures of only the soil + humic acid treatment are
presented. Parameters for the stems are also not presented, due to the small
difference from those of the leaves.
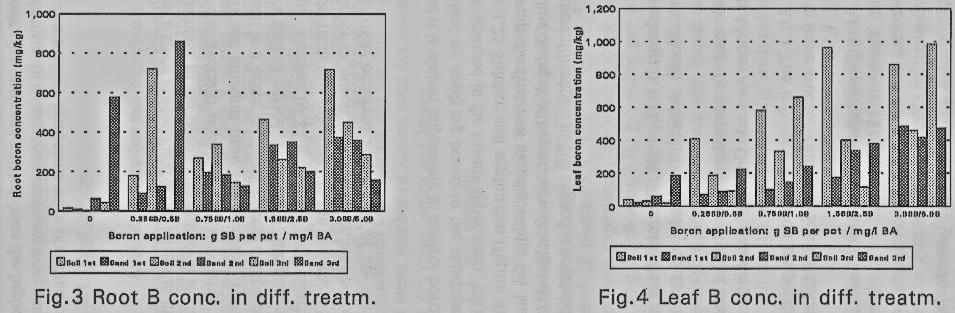
The line graphs in figures I and
2 represent combined means for all three substrates and show a linear increase
in the boron concentration from the lower to the higher boron concentrations in
the medium. In general, root and leaf dry mass show a clear tendency towards a
parabolic distribution with the optimum between the second and third treatments
(figures I & 2). From the first to the third harvest, the optimum shifted
from the higher towards the lower boron concentrations in the substrate. In
substrates containing more than I mg/1 B or 0.7 g Solubor/container, severe
boron toxicity symptoms were observed on the leaves.
4. Discussion
Boron is taken up by the
avocado roots against a steep gradient and accumulates in the above-ground
organs of the plant. The linear increase in the boron concentration in the
leaves and roots (figures 1 - 4) shows that the avocado does not seem to have a
mechanism to stop the boron uptake once sufficient amounts have accumulated in
the leaves or other organs. In the soil medium, which is a better buffered system
compared to the sand, toxic boron levels were reached in a shorter period. In
figure 4, the decline in the leaf boron concentration in the second soil
treatment (0.25 g Solubor/pot), clearly shows that avocado plants 'pump' out
the boron from the substrate. In the comparable sand medium, where there was a
continuous supply of boron, an increase in leaf boron concentration occurred
while toxic levels in the roots occurred at the time of the third harvest.
Results from this experiment
clearly show that it is almost impossible to control boron levels in the
vegetative parts of the avocado plant by applying boron to the substrate.
Although optimal leaf and root dry masses were obtained in the lowest
application, toxic boron levels occurred in the roots. In the single 0.25
Solubor soil application (figure 4), too much boron was absorbed by the roots
during the first two months, while the leaf boron started to decline.
Continuous or regular low dosages of boron, as was achieved in the sand
culture, may solve the problem, but more information in this regard is
required.
References
Coetzer, L.A.,
Robbertse, P.J. and Janse van Rensburg, B.P.H., 1993. The role of boron in
avocados: Theory, practice and reality. South African Avocado Growers'
Association, Yearbook 16: 2-4.
Coetzer, L.A.,
Robbertse, P.J., Barnard, R.O. and Tomer, E., 1994. Uptake and transport of
boron in avocado seedlings. South African Avocado Growers' Association,
Yearbook 17: 95-98.
Robbertse, P.J.
and Coetzer, L.A., 1988. (In Mr.) The influence of boron on pollen germination,
pollen tube growth and fruit set in some avocado cultivars. South African
Avocado Growers' Association, Yearbook 11: 65-67.
Robbertse, P.J. and Coetzer,
L.A., 1990. (In Afr.) Boron uptake by avocado leaves. South African Avocado
Growers' Association, Yearbook 13: 37.
Robbertse, P.J., Coetzer,
L.A. and Janse vanVuuren, B.P.H., 1992. Boron uptake by avocado. South African
Avocado Growers' Association, Yearbook 15: 89-93.
Vasil, I.K., 1963. Effect of
boron on pollen germination and pollen tube growth. In: Linskens, H.F. (ed.)
Pollen physiology and fertilization. North Holland, Amsterdam.