Proceedings of The World Avocado Congress III, 1995
pp. 223 - 226
IN
VITRO SHOOT PROLIFERATION IN AVOCADO (Persea americana Mill.) INDUCED
BY CPPU.
M. Castro, E. Oyanedel and R. Cautín
Facultad de Agronornía
Universidad Católica de Valparaiso
Casilla 4-D, Quillota
Chile
Keywords: tissue culture, browning
Abstract
Efficient methods of clonal
propagation are highly required by the avocado industry, in order to increase
the use of rootstocks with salinity and Phytophthora resistance. Tissue
culture techniques in avocado are difficult because the tissues have the
tendency to produce browning and later necrosis. It is scarce the rooting
potential in vivo and in vitro as well.
Active growing shoots from
summer, fall and spring vegetative flushes, were excised and kept in a solution
of 500 mg/l ascorbic and citric acids, with 50% v/v of ice. WPM medium was
used, in assays for types of explant; season; surface sterilization;
antioxidants and growth regulators, with the cultivars 'Lula' and 'Velvick'.
The enhanced protocol consist of using axillary buds, surface sterilized with 95% ethanol for 5 seconds, followed by 0. 5% sodium hypochlorite plus 0.1 ml/l Tween 20 and five rinses with antioxidant solution. Once inoculated, cultures were kept, first in darkness and then, under increasing light intensities (500, 1500 and 3500 lux) for equal periods of seven days each.
Stage I and 11 were carried
out in the same medium (using 0.2% agar, 0.2% gelrite, 60 mg/l ascorbic acid).
The optimal condition for 'Lula' and 'Velvick' were 0.5 and 0.1 mg/1 CPPU
[N-(2-chloro-4-pyridfl)-N-phenylureal, obtaining up to 92% of establishment
rate and 1.4 shoots per explant. Lower levels of browning were detected when
using CPPU, in comparison to TDZ [1-phenyl-3-(1,2,3-thiadiazol-5-yl) urea] and
BA. No seasonal effect was detected, as long as the mother plant presented
vegetative growth.
1.
Introduction
Current clonal propagation in
avocado is carried out by the method of double grafting (Brokaw, 1987), in an
expensive and very time consuming process. Limited success has been obtained in
cutting propagation and tissue culture. Juvenile tissues have been used in
vitro, obtaining roots from microcuttings (Pliego-Alfaro et al., 1987). Use
of juvenile material has the disadvantage of genetic variation by the
previously required seed propagation of stock plants. An alternative method can
be used, by grafting adult material on seedling stocks in vitro, which
may increase the rooting potential of the scion (Pliego-Alfaro, 1988). This is
the first report in regeneration with adult material in 'Lula' and 'Velvick'.
2. Material and Methods
Active growing terminal
shoots (5-7 cm) were selected from 4-year-old trees of 'Velvick', and
8-year-old 'Lula'. Vegetative material from summer, fall and spring flushes
were used. Explants were kept in 500 mg/l ascorbic acid and citric acid, plus
50% v/v ice. Axiltary buds were excised under the same acids. WPM medium (Lloyd
and McCown, 1981) was used, with (mg/1) 0.4 thiamine, 500 casein hydrolysate,
80 glutarnine, 100 myo-inositol, 1000 polyvinylpolypyrrolidone, 60 ascorbic
acid, and 30.000 sucrose, solidified with 0.2% agar + 0.2% gelrite. Thirty
replicates were used in each experiment. Cultures were kept in the dark for 7
days, and progressive illumination (500, 1500, 3500 lux), every 7 days, at 25+2ºC
and 16 h of fight. Explants were surface sterilized with 95% ethanol for 5 sec,
and 0.25% sodium hypochlorite + 0.01 mg/l tween 20 for 20 min. Different
concentrations of BA, IBA, GA3, CPPU [N-(2-chloro-4-pyiidil)-N-phenylurea] and
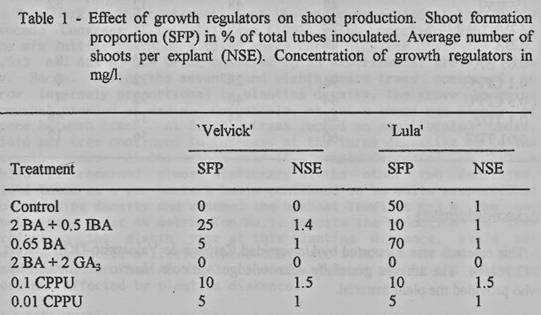
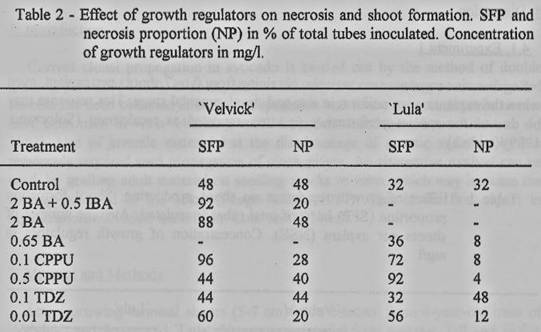
TDZ [1-phenyl-3-(1,2,3- thiadiazol-5-yl) urea] were used (tables 1 and 2),
using methods reported by Dalsaso and Guevara (1989), Phego-Alfaro et al.
(1987) and Pliego-Alfaro (1988). Results were obtained after 90 days in
culture, subculturing afterwards in the same media.
3. Result
Different response was obtained
between the cultivars. Highest shoot formation was observed with 0.65 mg/I BA
in 'Lula!, while 'Velvick' presented a recalcitrant performance in all the
treatments (table 1). Nevertheless, highest proliferation rate was produced by
0. 1 mg/l CPPU in both cultivars. Best treatments for each cultivar were
repeated (table 2), to compare with TDZ and CPPU.
In the second experiment, the
number of shoots per explant was always one. Differences were observed in the
necrosis rate (table 2). In 'Lula', CPPU produced the highest rate of shoot
formation and the lowest necrosis.
4.
Discussion
4. 1. Experiment I
Regenerative response was
variable, obtaining from 0 to 7 shoots per explant, even when the explants were
uniform in size and developmental stage. This response may be due to the
species performance in vitro, reported as recalcitrant (Solorzano, 1989)
(table 1).
4.2. Experiment 2
In 'Velvick', the effect of
BA on shoot formation was isolated from IBA, but a different rate of necrosis
was observed, which may indicate that the auxin is required for normal
development, when BA is present.
In addition to an increased
proliferation, the effect of CPPU was to prevent browning. Many explants of
'Velvicle and 'Lula! were growing for 4-6 weeks and then shifted to necrosis.
This effect may be produced by increasing the sink activity in the tissue, or
by inhibiting the activity of polyphenoloxidases. CPPU and TDZ induced
adventitious shoots, originated from epidermal tissue at the distal portion.
Shoots produced in BA treatments arose from pre-formed terminal or lateral
buds. Subcultured shoots presented necrosis and defoliation;. survival rate
were 20 to 45%, even when using low temperature, antioxidants and darkness to
prevent browning. No difference was observed in the survival rate between
shoots from different treatments.
Acknowledgement
This research was supported
by Universidad Católica de Valparaiso-DGIP, Grant 242.761/94. The authors
gratefully acknowledge Agricola Huerto California Ltd., who provided the plant
material.
References
Brokaw, W.H., 1987. Avocado
clonal propagation. Proc. Intern. Plant Prop. Soc. 37:97-103.
Dalsaso, L., and Guevara, E.,
1989. Multiplicación clonal in vitro del aguacate (Persea americana) cv.
Fuerte. Agronomía Costarricense 13:61-71.
Lloyd, G., and McCown, B.,
1981. Woody plant medium. Proc. Intern. Plant Prop. Soc. 30:421.
Pliego-Alfaro, F., Encina,
C.L., and Barceló-Muñoz, A., 1987. Propagation of avocado rootstocks by tissue
culture. South African Avocado Growers’ Assoc. Yearb. 10:36-39.
Pliego-Alfaro, F., 1988.
Development of an in vitro rooting bioassay using juvenile- phase steam
cutting of Persea americana. J. Hort. Sci. 63:295-301.
Solorzano, D., 1989.
Propagation in vitro of rootstocks of avocado. Calif. Avocado Soc.
Yearb. 73:149-151.