Proceedings of The World Avocado Congress III, 1995 pp. 340 – 343
CHANGES IN GLYCOSIDASE
ACTIVITIES WITH ETHYLENE PRODUCTION DURING FRUIT SOFTENING IN AVOCADO
Akira Tateishi and Hiroaki Inoue
College
of Agriculture and Veterinary Medicine
Nihon
University
Fujisawa Kanagawa Japan
Abstract
(α-Arabinofuranosidase
and β-galactosidase activities were assayed during fruit development and
ripening in avocado (Persea americana Mill. cv. Fuerte) fruit.
(α-Arabinofuranosidase activity increased drastically with ethylene production
at 4 days after harvest, although weak activity was detected during fruit
development. This suggests this enzyme is relating to fruit softening. On the
other hand β-galactosidase activity was detected during fruit development
and changed slightly with ripening.
Additional
index words
cell
wall, ripening, α-arabinoftu-anosidase, β-galactosidase
1. Introduction
Fruit softening is closely
connected with cell wall modifications caused by some cell wall degrading enzymes
(Fischer and Bennett, 1991). Polygalacturonase is one of the important enzymes
in relation to tomato fruit softening. Recently it was suggested that
polygalacturonase was not sole determinant of fruit softening (Smith et al.,
1988, Giovannoni et al., 1989). In avocado, cellulase which is synthesized at
the onset of ripening (Christoffersen et al, 1984) is well discussed and was
suggested to closely relate to the fruit softening. Cellulase purified from avocado fruit degraded the substrates
containing (1-4)-β-glycosyl linkages although it did not hydrolyze the
cellulose polymer from mature avocado cell wall (Hatfield and Nevins, 1986).
Further, it released arabinose and galactose in incubation with the cell wall
from unripe fruit (Hatfield and Nevins, 1986).
The release of galactose and
arabinose from pectic side chains caused by glycosidases during softening was
detected in many kinds of fruit and is an important event in fruit softening
(Redgwell et al., 1992, Dawson et al., 1992). β-Galactosidases were also
purified from avocado fruit and its role on pectin solubility was discussed
(Ian De Veau et al., 1993). However, there are little information of other
glycosidases. Therefore, we investigated the alteration of β-galactosidase
and α-arabinofuranosidase activities during fruit development and ripening
to elucidate the role of those glycosidases in avocado fruit softening.
2. Material & Methods
2.1 Plant material
Avocado fruits (cv. Fuerte)
were harvested periodically (August to March) in Numazu, Shizuoka prefecture in
Japan. Mesocarp tissue of fruit was sliced and freezed before using. The fruits
harvested in November were stored at 25 °C and then mesocarp tissue was sliced
and freezed before using.
2.2 Measurement of ethylene
production and fruit firmness
Ethylene production was
measured by gas chromatography and fruit firmness was measured by Handy HIT
(Fujihira Co. Ltd. Japan; nondestructive handy hardness meter, range
500-800gf).
2.3 Assay of glycosidase
activities
Enzyme extraction procedure
was carried out at 4°C. Tissue was homogenized in 0.1 M K-phosphate buffer (pH
6.5) containing 30 mM 2-mercaptoethanol and 0.1% (w/v) sodium-L-ascorbate. The
homogenate was centrifuged at 10,000*g for 20 min. The precipitate was
resuspended in 10 mM K-phosphate buffer (pH 6.5) containing 5 mM
2-mercaptoethanol and centrifuged at 10,000*g for 20 min. This step was
repeated three times and the precipitation was suspended in the same buffer.
The supernatant and precipitate were dialyzed separately against the same
buffer and defined as buffer soluble and cell wall bound fractions,
respectively.
α-Arabinoftiranosidase and β-galactosidase activities were
assayed using p-nitrophenylglycosides as substrate.
3. Results and discussion
3.1 Ethylene production and
fruit softening
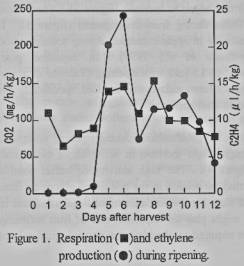
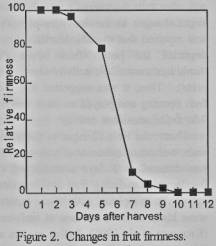
Ethylene production was
detected at 4 days after harvest (figure 1), then the fruits reached the best
soft to eat at 7 days (figure 2).
3.2
α-Arabinofuranosidase activity
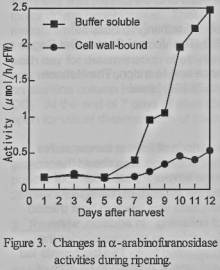
Cell wall bound
α-arabinofuranosidase and buffer soluble enzyme activities increased
drastically after the peak of ethylene production. The fruit reached to edible
soft after their α-arabinofuranosidase activities began to increase
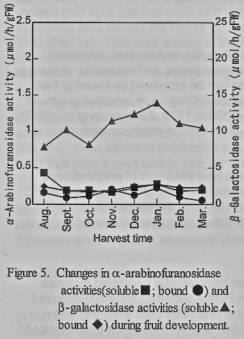
(figure 3). There were slight changes in the activities of both forms during
fruit development (figure 5). It was reported that (α-arabinofuranosidase
activity in apple increased during softening and degraded the pectin from apple
(Yoshioka et al., 1995). In Japanese
pear, α-arabinofuranosidase activity also increased to 15-fold with
ripening (Tateishi et al., in press). Thus, it was suggested that
α-arabinofuranosidase was induced specifically for fruit ripening and
played an important role in fruit softening.
3.3 β-Galactosidase
activity
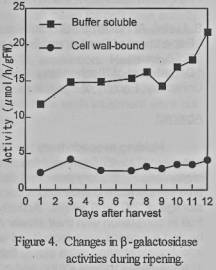
There was little changes in
the activity of β-galactosidase during ripening compared with
α-arabinofuranosidase activity although slight increase in activities of
both form was detected at 3 days after harvest (figure 4). The high activity of
buffer soluble β-galactosidase during fruit development may be related in
the release of glycoside from aglycon in cytoplasm. As it was reported that
there were isoforms of β-galactosidase in some kinds of fruit, and one of
isoforms might play an important role of fruit softening (Kitagawa et al.,
1995), further studies are required to elucidate the physiological role of this
enzyme in fruit softening.
4. Acknowledgements
The authors are very grateful
to Prof S. Yamaki, Nagoya University, for helpful discussions and critical
reading of the manuscript.
5.
References
Dawson, D.M., Melton, L.D.
and Watkins, C.B. 1992. Cell wall changes in nectarines (Prunus persica). Plant
Physiol. 100: 1203-1210.
Fischer, R.L. and Bennett,
A.B., 1991. Role of cell wall hydrolases in fruit ripening. Annu. Rev. Plant
Physiol. Plant Mol. Biol. 42:675-703.
Giovannoni, J.J., DellaPenna,
D., Bennett, A.B. and Fischer, R.L., 1989. Expression of a chimeric
polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit
results in polyuronide degradation but not fruit softening. Plant Cell 1:53-63.
Ian De Veau, E.J., Gross,
K.C., Huber, D.J. and Watada, A.E., 1993. Degradation and solubilization of
pectin by β-galactosidases purified avocado mesocarp. Physiol. Plant.
87:279-285.
Kitagawa, Y., Kanayama, Y.
and Yamaki, S., 1995. Isolation of β-galactosidase fractions from Japanese
pear: Activity against native cell wall polysaccharides. Physiol. Plant.
93:545-550.
Redgwell, R.J., Melton, L.D.
and Brasch, D.J., 1992. Cell wall dissolution in ripening kiwifruit (Actinidia
deliciosa). Plant Physiol. 98:71-81.
Smith, C.J.S., Watson, C.F.,
Ray, J., Bird, C.R., Morris, P.C., Schuch, W. and Grierson, D., 1988. Antisense
RNA inhibition of polygalacturonase gene expression in transgenic tomatoes.
Nature 334:724-726.
Tateishi, A., Kanayama, Y.
and Yamaki, S., 1995. α-L-Arabinofuranosidase from the cell wall of
Japanese pear fruit. Phytochemistry (In press)
Yoshioka, H., Kashimura, Y.
and Kaneko, K., 1995. β-D-Galactosidase and α-L-arabinofuranosidase
activities during the softening of apples. J. Japan. Soc. Hort. Sci.
63:871-878.