Proc. of Second World Avocado Congress 1992 pp.75-78
Timing of Phosphonate Trunk
Injections for Phytophthora Root Rot
Control in Avocado Trees
Anthony W. Whiley,
Jack B. Saranah, and Peter W. Langdon
Maroochy
HRS,
Philip A. Hargreaves, Ken G. Pegg, and
Leslie J. Ruddle
Department
of Primary Industries,
Abstract. Avocado trees, cv. Hass, were trunk-injected
with 20% phosphonic acid, formulated as potassium phosphonate, at various stages of tree phenology
and the dynamics of translocation to the roots studied. Phosphonate
was detected in the leaves 24 h after trunk injection with the concentration
peaking (60-80 mg/kg) within the first 10 days. However, the rate of accumulation
and final concentration of phosphonate in the roots
was dependent on the time of injection in relation to the sink/source status of
the leafy shoots. Trunk injection at the beginning of the spring growth, when
renewal shoots were strong sinks, resulted in phosphonate
root con- centrations of < 9 mg/kg which peaked at
about 45 days after treatment. When phosphonate
injections were given after the sink/source transition of the spring shoots,
root concentrations increased to > 28 mg/kg at about 60 days after treat- ment. Injections of potassium phosphonate
given during the period of summer shoot growth gave similar concentrations of phosphonate in the roots to the treat- ment
given at spring shoot maturity.
Phytophthora cinnamomi
Materials and Method
The trees selected for the experiment were healthy
twelve-year-old 'Hass' grafted to Velvick seedling
rootstock, and had never been injected or sprayed with phosphonate
fungicides. The trees were growing in a commercial orchard in subtropical
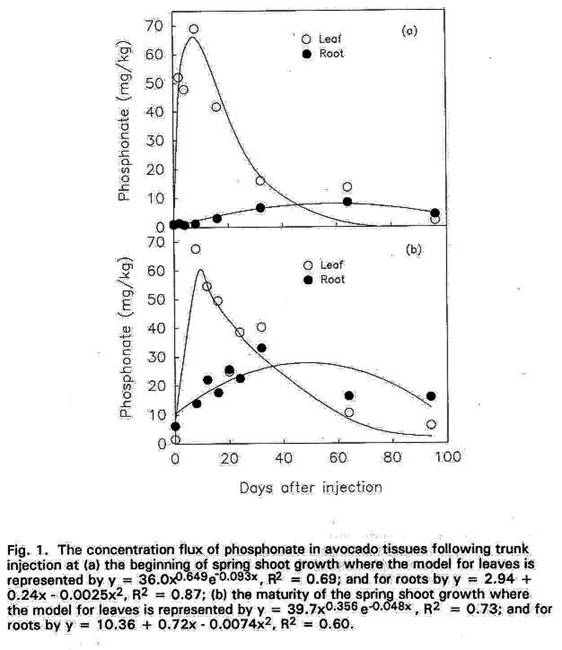
The concentration flux of phosphonate
in leaves for the 98 days it was monitored was fitted to the model derived by
Wood (1967) where y = axb e-cx. Regression analysis were
used for the concentration flux in roots.
Results
After each injection the subse- quent rise in leaf phosphonate
concentration was rapid, reaching a peak after 10 to 12 days of between 60 to
70 mg/kg (Fig. 1). This was followed by a decline in leaf phosphonate
to < 5 mg/kg 80 days after injection. Similarly the root phosphonate concentrations increased soon after trunk
injection but at a slower rate and the maximum concentrations measured were
considerably lower than in leaves (Fig. 1). Roots of those trees injected at
the beginning of the renewal growth in spring, reached a maximum of ≈ 9.0
mg/kg phosphonate about 45 days after treatment.
However, the roots of trees treated when all leaves on spring-grown shoots were
fully expanded had a phosphonate concentration of ≈
28 mg/kg about 60 days after injection. The experiment was repeated the
following spring using another set of trees and similar results were obtained
(data not presented).
Discussion
While some controversy exists in the literature on the mode
of action of phosphonates in disease prevention it is
clear that the organ targeted for protection must receive a minimum
concentration of the fungicide for the mechanism to be effective. For efficient
management of the technology, the grower should know when to inject his trees
to maximize the translocation of phosphonate to the
roots during the time when disease pressures are greatest. Our research has
shown that trunk-injected phosphonate begins
accumulating the roots of avocado trees within a few days of injection
confirming previous results of Schutte et at. (1988). However, it is clear that the
time of injection in relation to tree phenology is
critical with respect to maximizing the translocation of phosphonate
to the roots. In actively growing trees, the maximum concentration of phosphonate to accumulate in the roots after injection at
the beginning of spring shoot growth (≈ 9.0 mg/kg), was significantly lower
than the minimum of 20 mg/kg thought to be required for protection against Phytophthora cinnamomi in
field grown trees (Pegg and Whiley, unpublished
data). However, in contrast up to ≈ 28 mg/kg of phosphonate
was measured in roots injected at the completion of spring shoot growth.
The efficiency of translocation of phosphonate
to the roots appears directly relate to the sink/source status of the leafy
canopy at the time of injection (Whiley, 1990; Pegg et
at., 1990). Following flowering there is a strong synchronization of shoot
growth in the canopy which effectively remains a sink for 40 days after
bud-break and injection should be avoided during this time. About 60 days after
the terminal bud of the indeterminant panicle begins
growing the shoots reach their maximum rate of photoassimilation
(Whiley, 1990). During this time there is an effective translocation of photoassimilates from the leaves to the roots (Whiley and
Schaffer, unpublished data). We believe that phosphonate
arriving in the leaves via the xylem stream, is more effectively translocated by the greater basipetal
mass-flow of photoassimilates at this stage of tree phenology.
Trunk injection during the summer when some new shoot growth
was occurring gave a similar accumulation of phosphonate
in roots to those of trees injected at the maturity of the spring growth (data
not presented). Sporadic shoot growth without synchrony of the whole tree
occurs within the canopy during summer. However, at anyone time there a sufficient
'source' leaves to enact efficient translocation of the fungicide to the roots
when tree are injected during this period.
Conclusions
By strategically timing the trunk injection of phosphonates into avocado trees the efficiency of translocation
of the fungicide to the roots can be increased by about 300%. This more
effective use of fungicide will be off considerable economic benefit to
producers requiring a root rot man- agement program.
In subtropical
We thank the Other
Fruits Committee of COD, UIM Agrochemicals (Aust.)
Pty Ltd, Albright and Wilson (Aust.) Ltd, and the
Australian Special Rural Research Council for their generous financial support.
Literature Cited
Darvas, J.M., J.C.
Toerien, and D.L. Milne. 1984. Control of avocado root rot by trunk injection with phosetyl-AI. Plant Disease 68: 691-693.
Lüttringer, M.
and L. De Cormis. 1985. Absorption, degradation et transport du phosethyl-AI et
de son métabolite chez la tomate. Agronomie. 5:423-430.
Pegg, K.G.,
A.W. Whiley, J.B. Saranah, and R.J. Glass. 1985. Control of Phytophthora
root rot of avocado with phosphorous acid. Austral.
Plant Path. 14:25-9.
Pegg, K.G., A.W. Whiley, and P.A. Harg- reaves.
1990. Phosphorous acid treatments control Phytophthora
diseases in avocado and pineapple. Austral. Plant
Path. 19:122-124.
Pegg, K.G., A.W.
Whiley, P.W. Langdon, and J.B. Saranah. 1987. Comparison of phosetyl-AI, phosphorous acid and metalaxyl
for the long-term control of Phytophthora root
rot of avocado. Austral. J. Exp. Agri.
27:471-474.
Schutte, G.C., T. Botha, J.J. Bezuidenhout, and
J.M. Kotzé. 1988. Distribution of phosphite
in avocado trees after trunk injection with phosphorous acid and its possible
response to Phytophthora cinnamomi. S. A. Avocado Growers'
Assn. Yrbk. 12:32-34.
Whiley, A. W.
1990. CO2
assimilation of developing fruiting shoots of cv.
Hass avocado (Persea
Whiley, A.W., J.B.
Saranah, B.W. Cull, and K.G. Pegg. 1988. Manage avocado tree growth
cycles for productivity gains. Qld. Agri. J. 114:29-36.
Wood, P.D.P. 1967. Algebraic model of the lactation curve in cattle. Nature
216:164-165.
Zentmyer, G.A. 1979. Effect
of physical factors, host resistance and fungicides on root infection at the
soil-root interface, p. 315-328. In: J.L. Harley and R. Scott Russell
(eds.) The Soil-Root Interface. Academic,